Oral Presentation 9th Modern Solid Phase Peptide Synthesis & Its Applications Symposium 2023
A Novel Allylic Cysteinyl Protecting Group for Expanding The Scope of One-Pot Multi-Segment Peptide Ligation (95354)
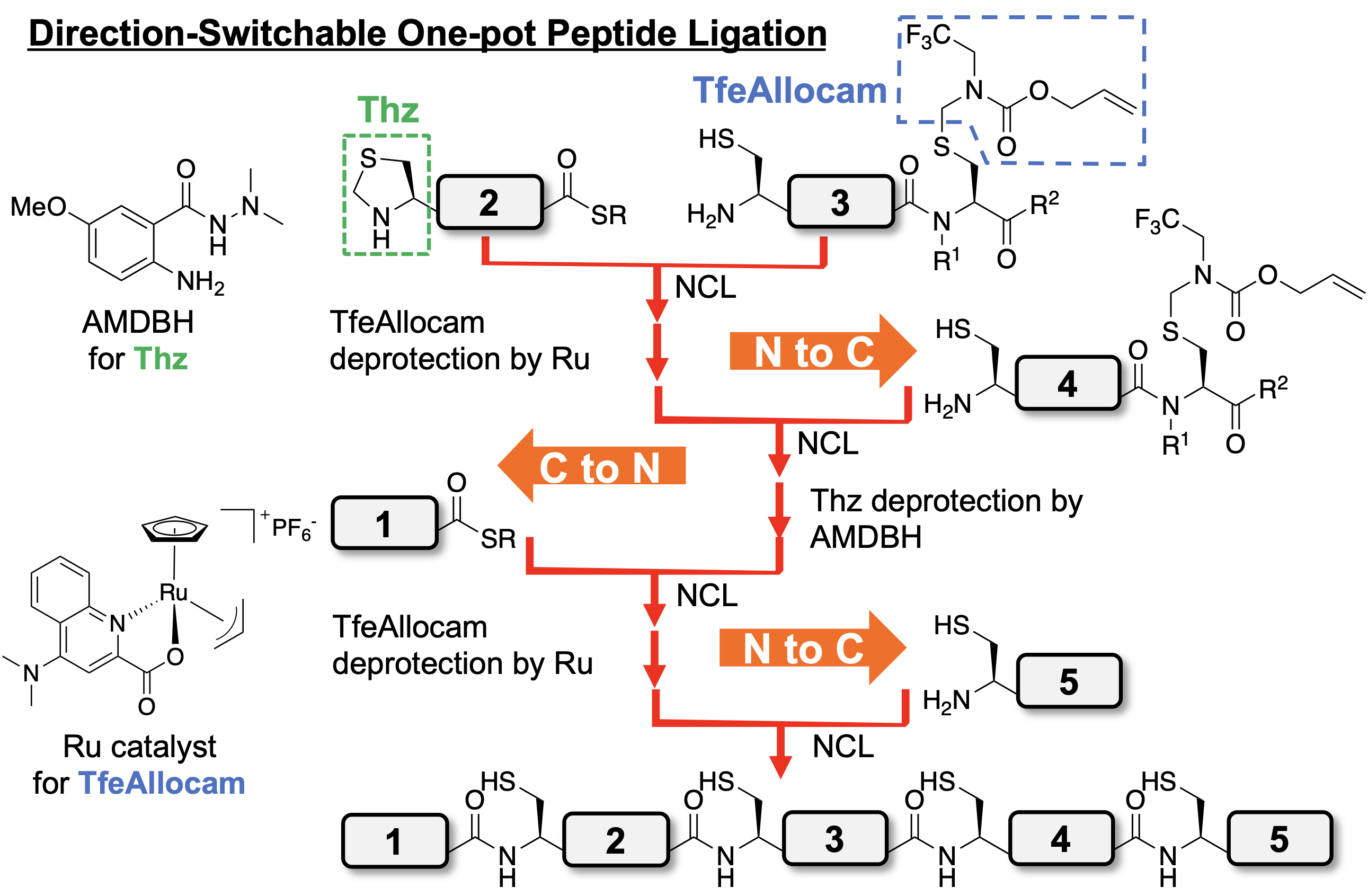
Chemical protein synthesis is a powerful strategy to produce uncommon proteins with multiple non-proteogenic amino acids. Peptide segment ligation is the essential step for chemical protein synthesis, and Native Chemical Ligation (NCL)[1] is the most prevalent and practical peptide ligation reaction. To streamline the procedures of chemical protein synthesis, many research groups have been developing methods for multiple peptide ligation in a one-pot manner. The reported methods of one-pot peptide ligation are roughly classified into two types according to the direction of Ligation: C-to-N and N-to-C one-pot peptide ligation. Notably, the direction of ligation is one of the decisive factors for the success of peptide segment condensation. However, there has been no report of peptide ligation in which the direction of ligation switches in a one-pot manner. Developing this new ligation system is a considerably challenging task because of the necessity of fully orthogonal one-pot peptide ligation systems for each C-to-N and N-to-C direction.
Herein, we report a newly synthesized TfeAllocam [N-(2,2,2-trifluoroethyl) allyloxycarbonylaminomethyl] as a cysteinyl protecting group. This allylic cysteinyl protecting group has full compatibility with Fmoc-SPPS and NCL conditions and is readily deprotected in around 10 min in the NCL buffer by adding Pd[2] or Ru catalyst[3]. The incorporation of TfeAllocam into an N-S acyl shift-mediated crypto-thioester enabled N-to-C one-pot ligation. The combination of this method and thiazolidine (Thz) deprotection mediated by 2-aminobenzamide derivative[4] resulted in the success of direction-switchable one-pot peptide ligation. Furthermore, we report the semi-synthesis of diubiquitinated histone H3 bearing trimethyl-lysine with this direction-switchable one-pot ligation method.
- Dawson, P. E.; Muir, T. W.; Clark-Lewis, I.; Kent, S. B. Science 1994, 266, 776.
- Kamo, N.; Hayashi, G.; Okamoto, A. Angew. Chem. Int. Ed. 2018, 57, 16533.
- Kamo, N.; Kujirai, T.; Kurumizaka, H.; Murakami, H.; Hayashi, G.; Okamoto, A. Chem. Sci. 2021, 12, 5926.
- Nakatsu, K.; Okamoto, A.; Hayashi, G.; Murakami, H. Angew. Chem. Int. Ed. 2022, 61, e202206240.